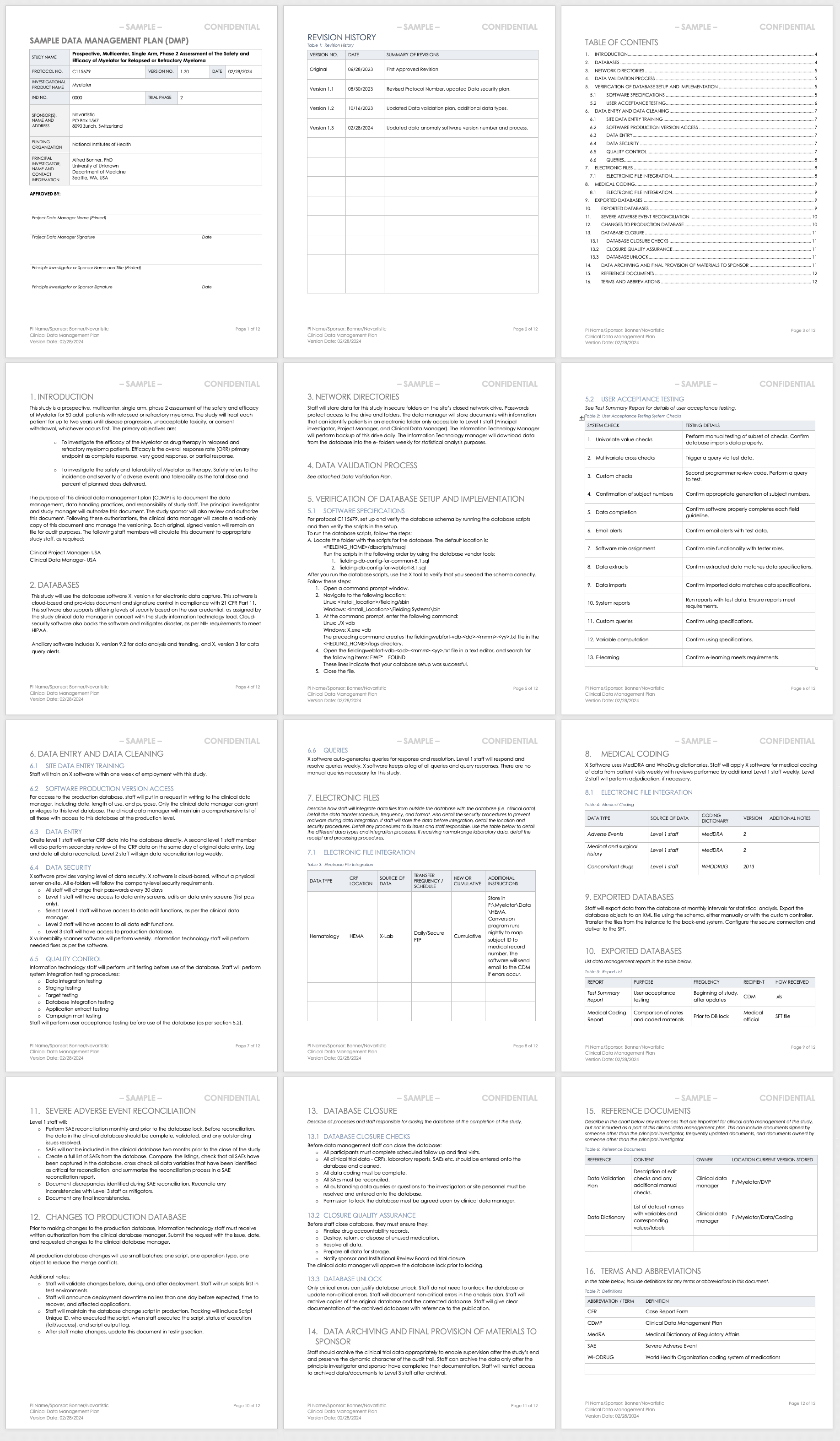
Papass clinical trial protocol: a multi-component school-based intervention study to increase acceptance and adherence to school feeding | BMC Public Health | Full Text

NHSBT/MRC Clinical Studies Unit Platelets for Neonatal Transfusion Study 2 (PlaNeT-2) A randomised controlled trial of platelet transfusion thresholds. - ppt download

Table 1 from Lumbar microdiscectomy and post-operative activity restrictions: a protocol for a single blinded randomised controlled trial | Semantic Scholar

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health

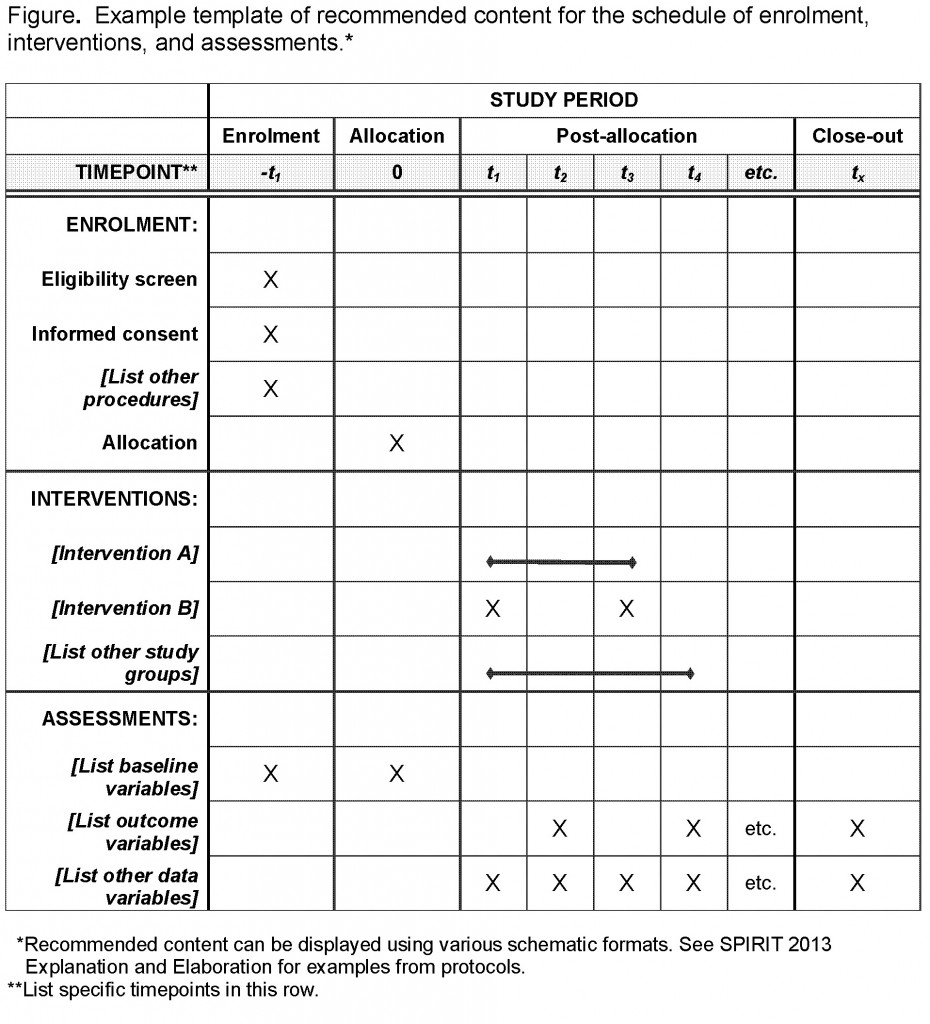
Schedule of enrolment, interventions, and assessments adapted from the... | Download Scientific Diagram

Protocol of the Definition for the Assessment of Time-to-event Endpoints in CANcer trials (DATECAN) project: Formal consensus method for the development of guidelines for standardised time-to-event endpoints' definitions in cancer clinical trials -

Effectiveness and safety of electroacupuncture for poststroke patients with shoulder pain: study protocol for a double-center, randomized, patient- and assessor-blinded, sham-controlled, parallel, clinical trial | Semantic Scholar

A double-blind randomized comparative clinical trial to evaluate the safety and efficacy of dendritic cell vaccine loaded with WT1 peptides (TLP0-001) in combination with S-1 in patients with advanced pancreatic cancer refractory

Schedule of Events. Example visit and assessment specification from a... | Download Scientific Diagram

David Cloutier Director, Research Center Management and Development Budgeting for Industry Sponsored Clinical Trials. - ppt download

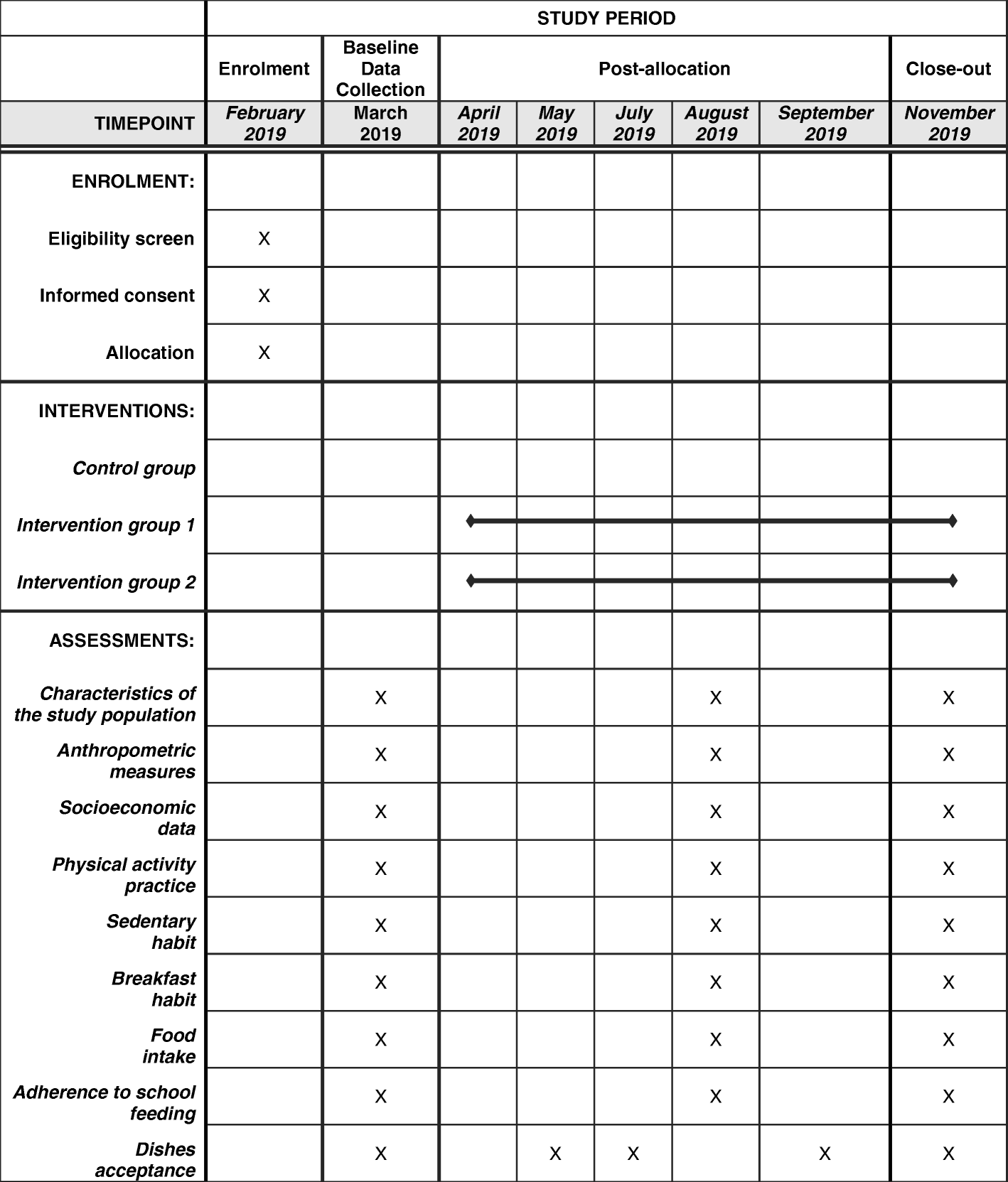
Schedule of enrollment, intervention, and assessment according to the... | Download Scientific Diagram