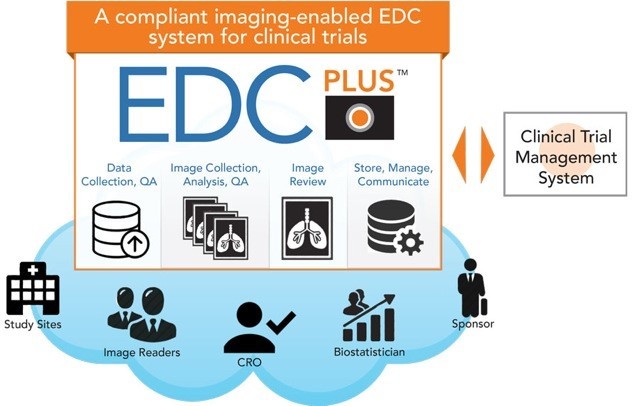
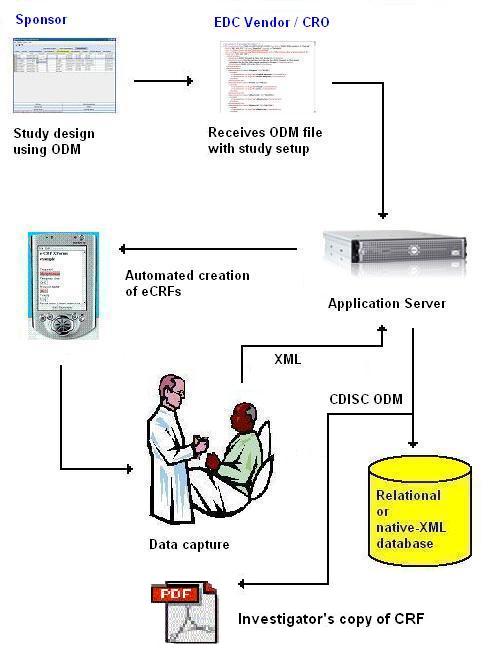
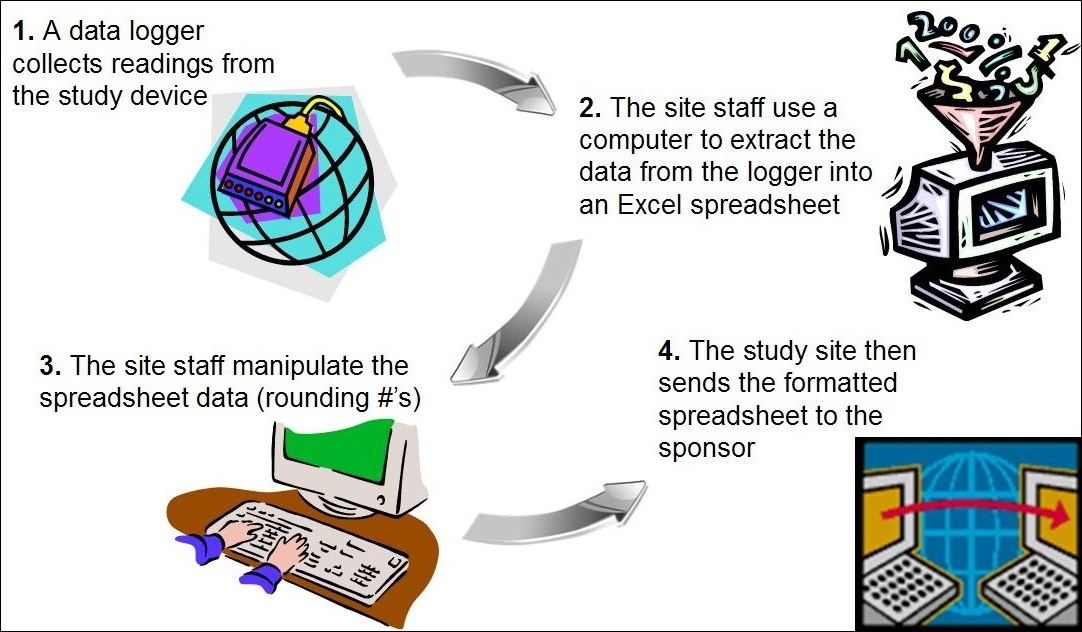
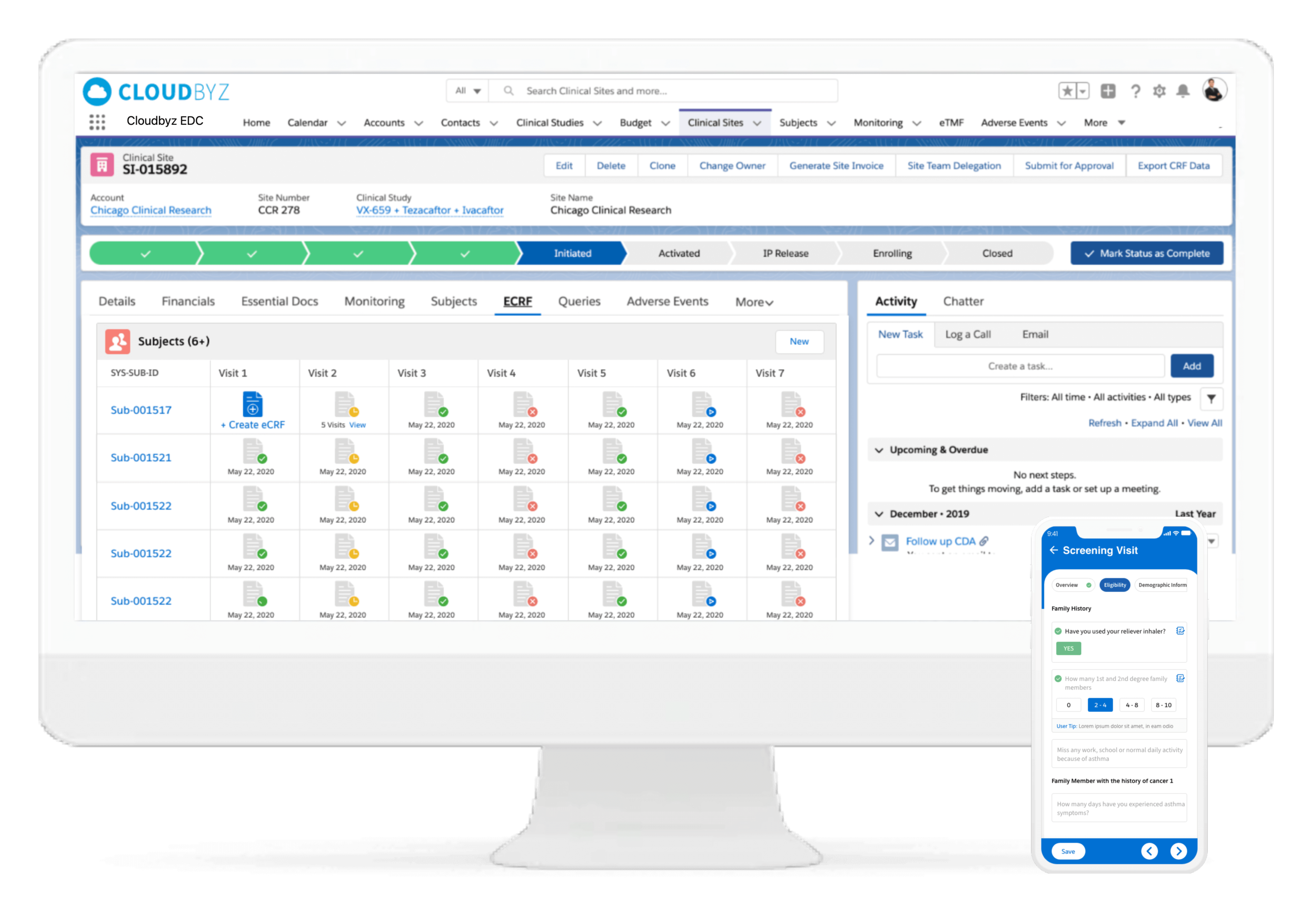
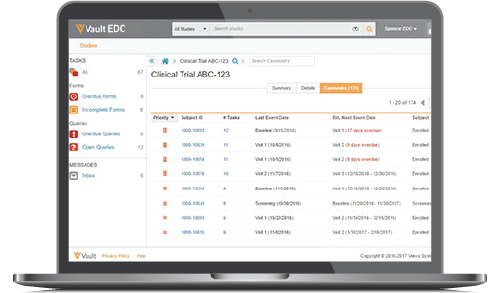
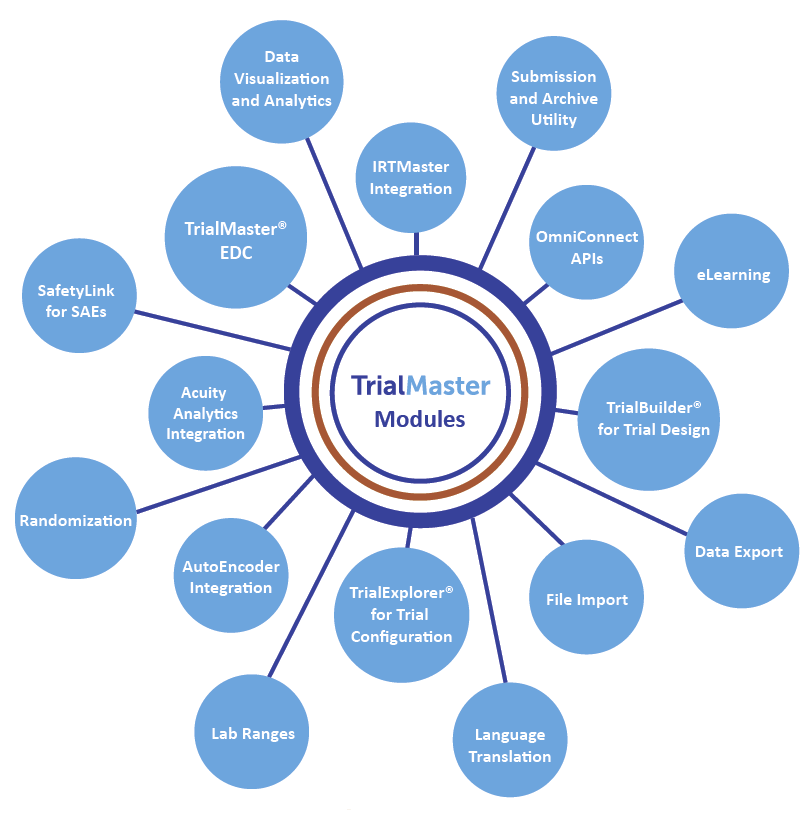
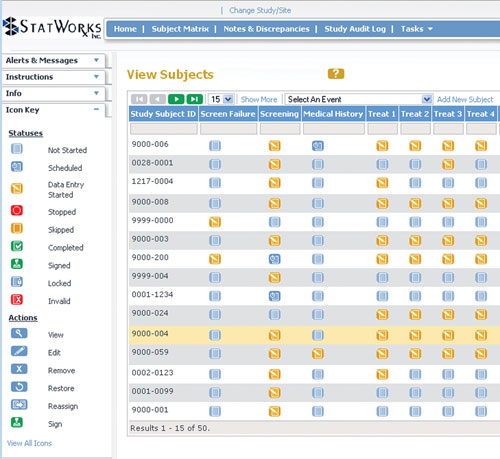
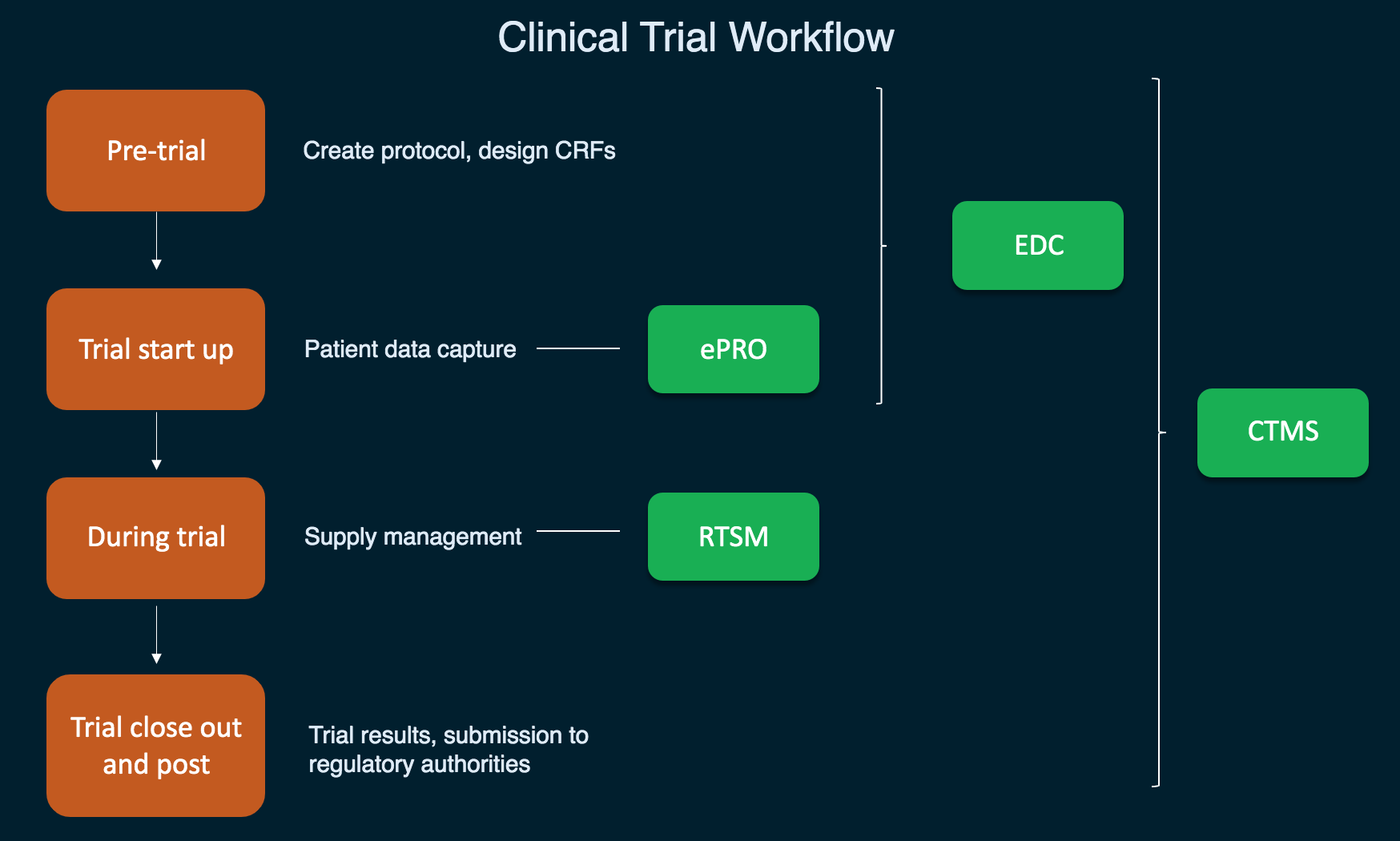
Pharma and biotecht Phase (I – II – III and IV) Clinical Trials | Electronic Data Capture (EDC) as a useful – ResearchManager

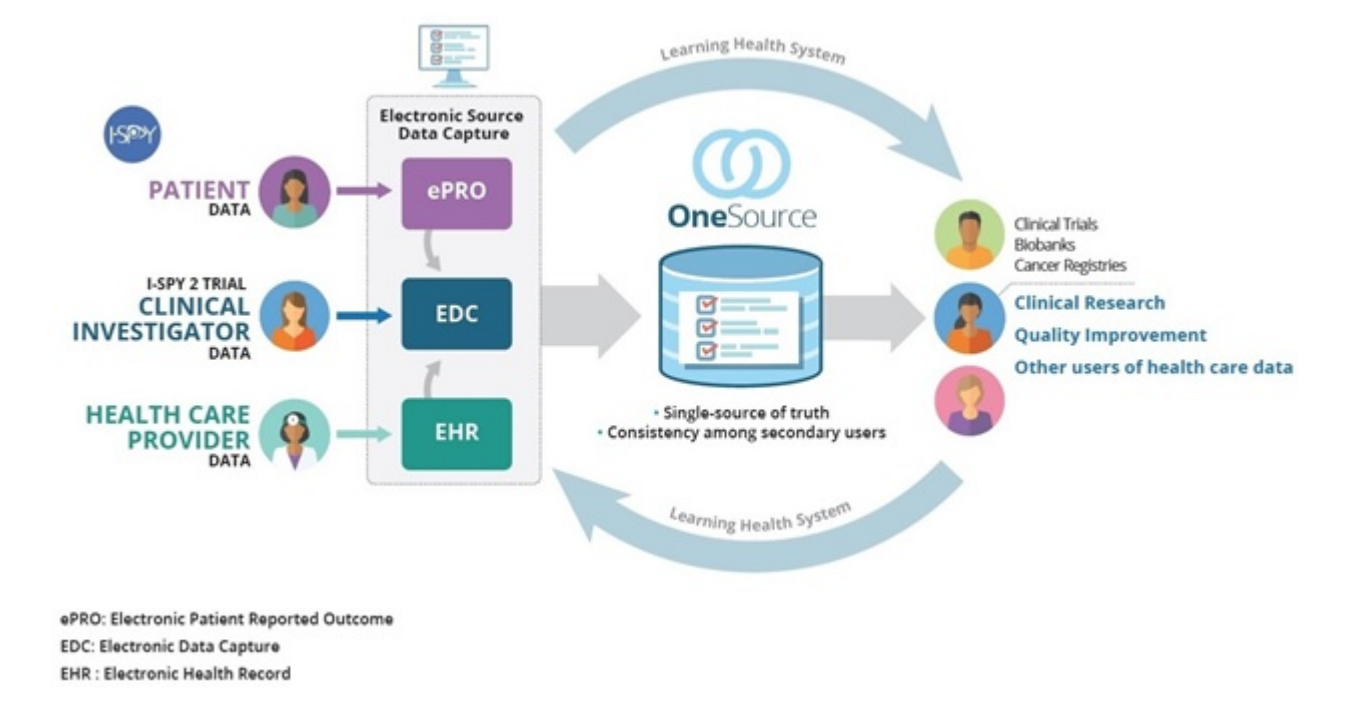
Electronic Source (eSource) vs. Electronic Data Capture (EDC): What's the Difference? - Clinical Research IO - CRIO