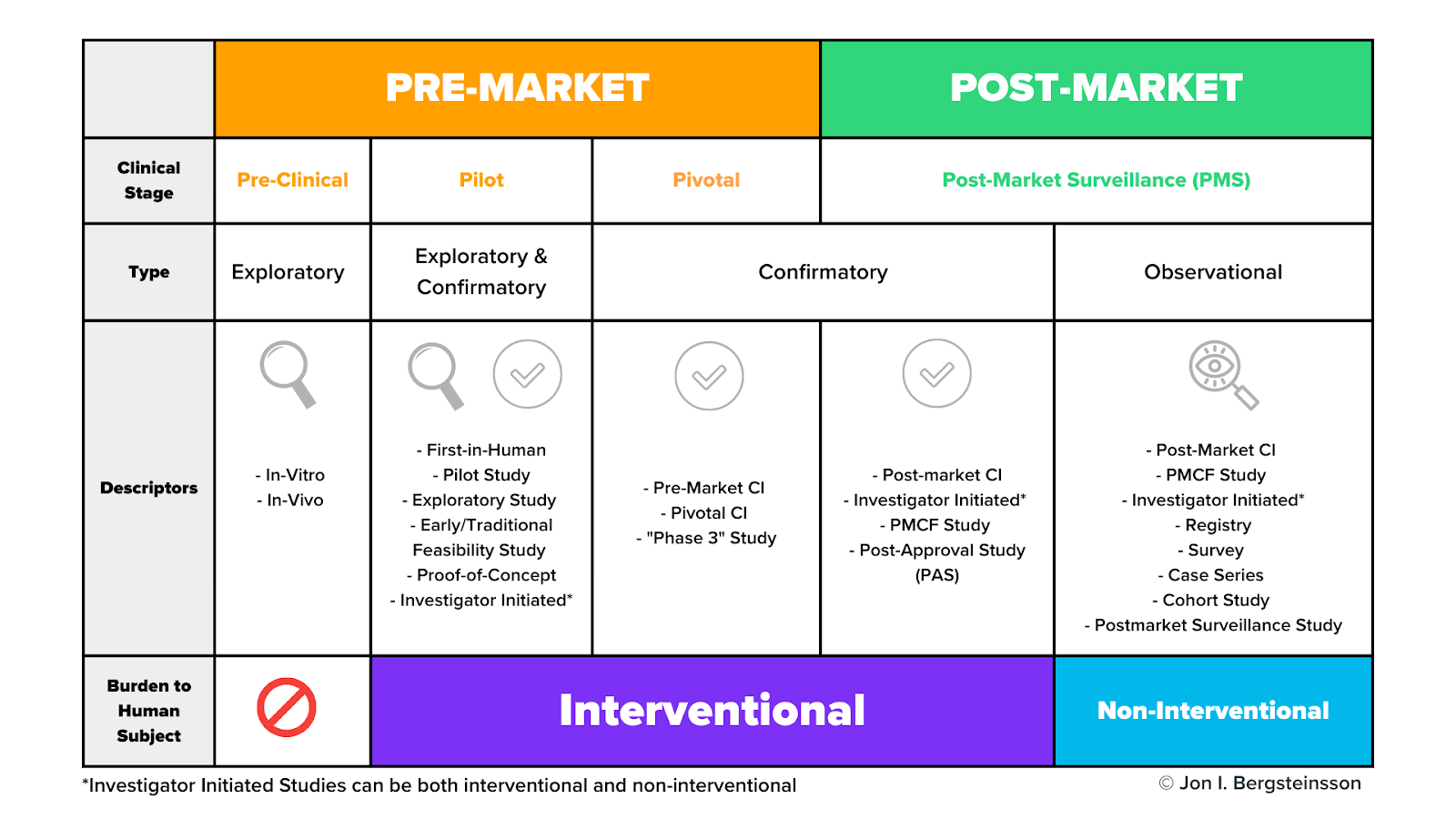
Distribution of Clinical trials on Medical Devices in Europe from 2015... | Download Scientific Diagram

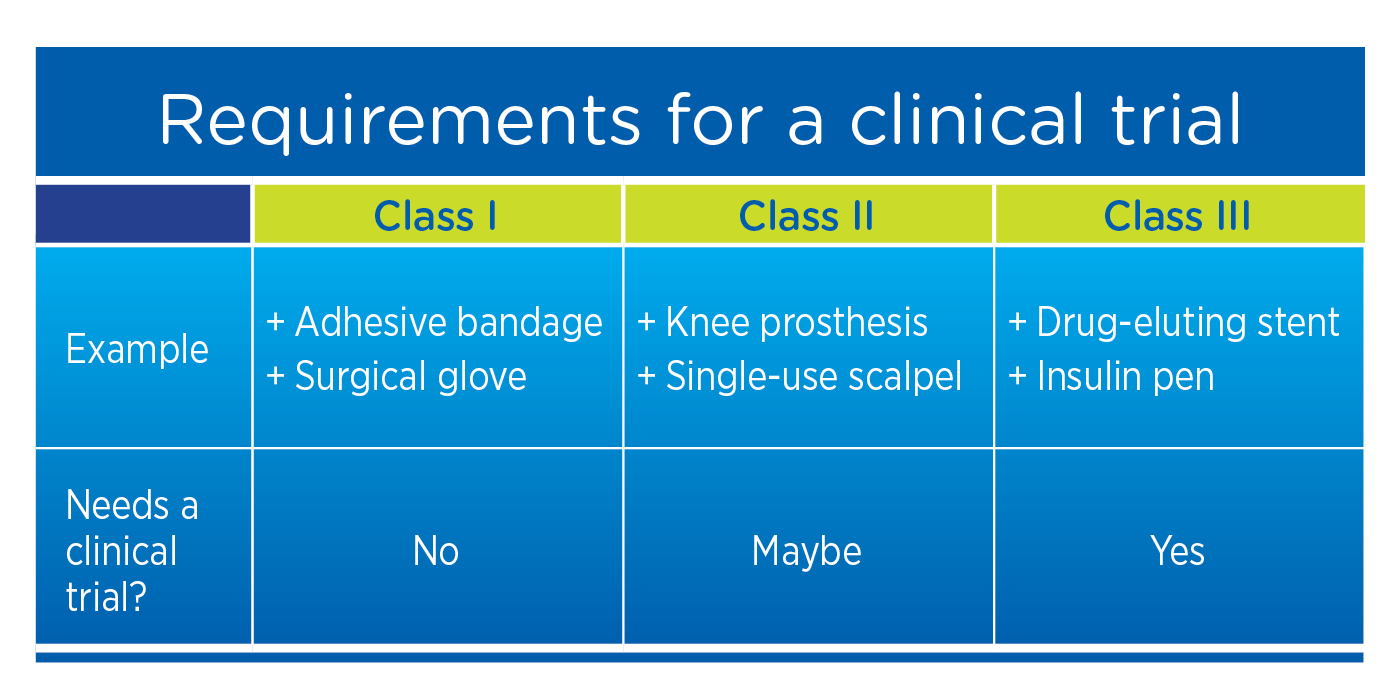
Outsourcing in Clinical Trials: Medical Devices Europe 2018 - Medical Technology | Issue 4 | November 2017

The medical device challenge in Europe (Part II): An acute demand for clinical experts and additional education
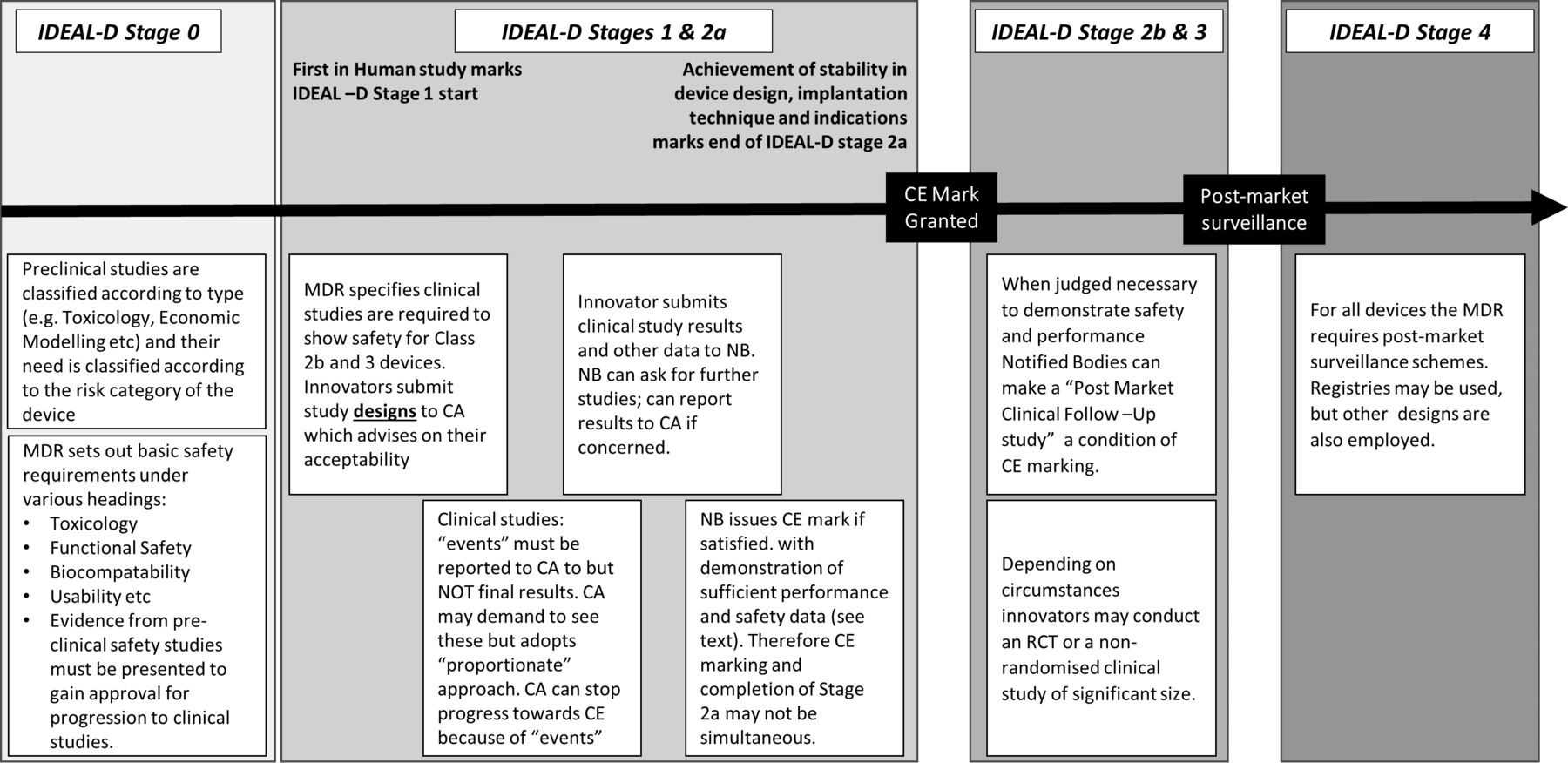
IDEAL as a guide to designing clinical device studies consistent with the new European Medical Device Regulation | BMJ Surgery, Interventions, & Health Technologies

Outsourcing in Clinical Trials: Medical Devices Europe 2018 - Medical Technology | Issue 4 | November 2017
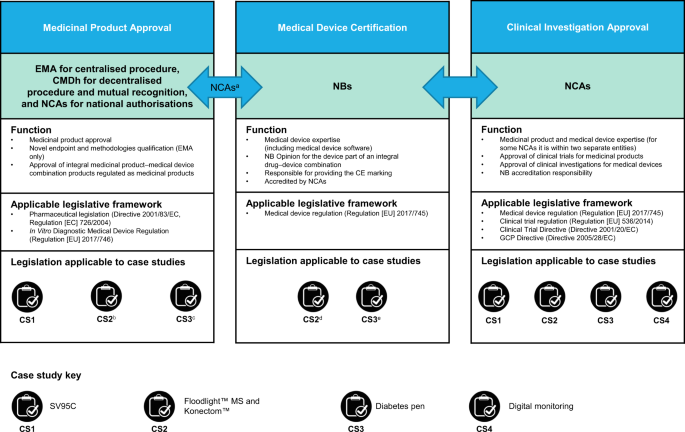
Evolving regulatory perspectives on digital health technologies for medicinal product development | npj Digital Medicine

ADVANCED CLINICAL TRIALS THE CLINICAL TRIAL PROCESS: IMPENDING CHANGES IN THE REGULATORY FRAMEWORK - ADVANCED CLINICAL TRIALS