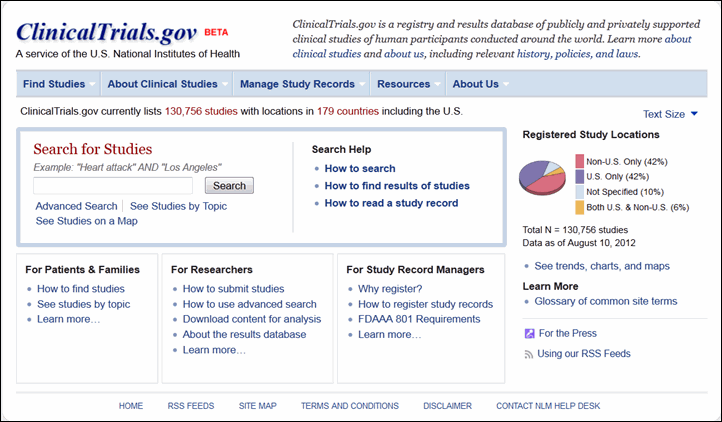
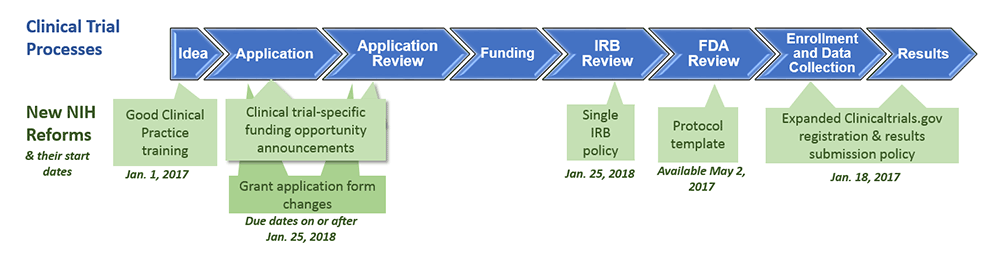
Celebrating 20 Years of ClinicalTrials.gov and Looking to the Future – NLM Musings from the Mezzanine

Los Angeles, California, USA - 25 January 2020: Clinical Trials website page. ClinicalTrials.gov logo on display screen, Illustrative Editorial Stock Photo - Alamy

Celebrating 20 Years of ClinicalTrials.gov and Looking to the Future – NLM Musings from the Mezzanine

Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis | The BMJ